Cosmetics, pharmaceuticals, and laundary are key end-user industries of Monoethanolamine
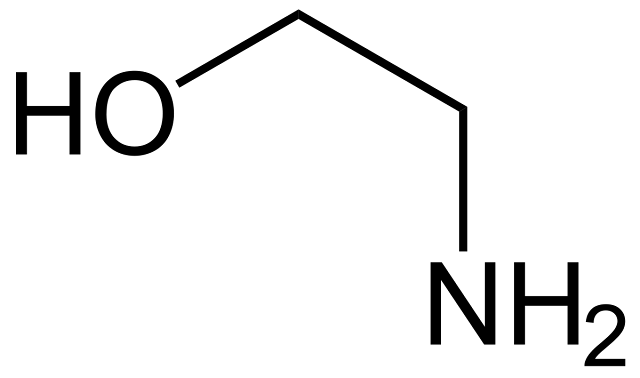
Monoethanolamine (MEA) is a colorless liquid that has two chemical properties: amine and alcohol. The amine group in this compound is derived from ethylene oxide and anhydrous ammonia. The production of this compound is exothermic in a reactor. Despite its odor, it is colorless, flammable, and corrosive. In addition to its industrial uses, it is found in cosmetics, pharmaceuticals, and detergents.
In
the post-combustion carbon capture process, an aqueous monoethanolamine
solution is frequently used. While the release and uptake of CO2 have been
extensively studied, intensive research is needed to understand the fundamental
chemical reactions.
Ethanolamine
(MEA) is the only ethanolamine found naturally in mammals. It is an
intermediate in the formation of phospholipids and choline. Unexposed humans
excrete MEA in their urine. In fact, women excrete it at a rate of 0.492
mg/kg/day. The FDA lists TEA on its list of indirect food additives, but this
does not necessarily mean that it's safe to consume in typical amounts.
A
clear viscous liquid with an ammonia-like smell, MEA is a common ingredient in
many cleaning products, including detergents and soaps. It is also used in
pharmaceuticals and corrosion inhibitors. Besides being used as an additive in
cosmetics and pharmaceuticals, Monoethanolamine
is also used in various industrial applications. It is used as a solvent for
fats and oils, as well as in the manufacture of ammonia. The compound also
promotes the alkalinization of water in power plant steam cycles. Aside from
its industrial uses, MEA is commonly found in everyday consumer products. It is
a widely used ingredient in non-pesticide agricultural chemicals, functional
fluids, anti-scaling agents, and corrosion inhibitors. While ethanolamine is
not toxic, however, overexposure can cause adverse health effects. It is not
recommended for human use, however, as it can be harmful if inhaled.



Comments
Post a Comment